Sign up for our monthly newsletter!
Get the latest information and inspirational stories for caregivers, delivered directly to your inbox.
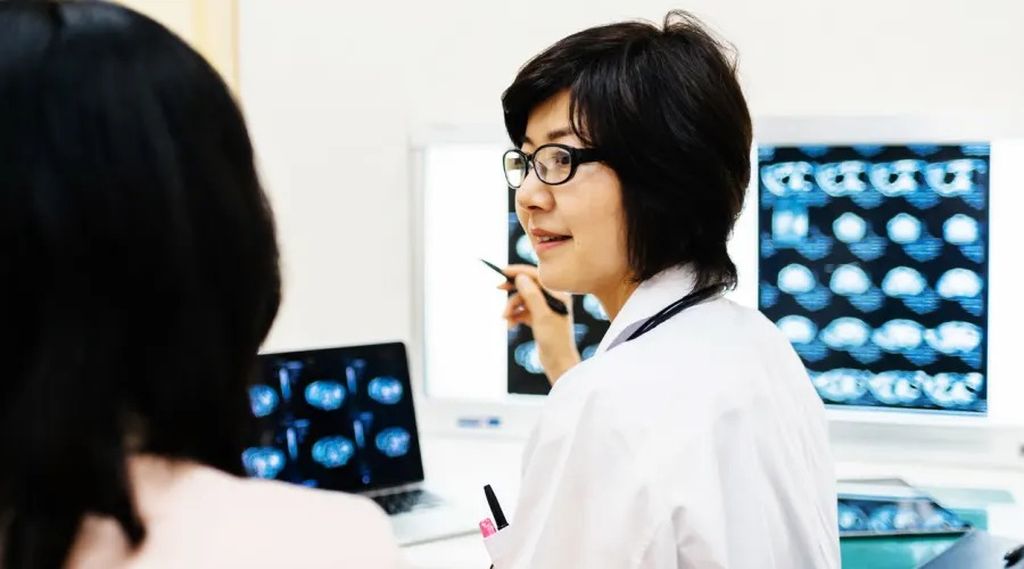
A Neurologist Answers Questions About the New Alzheimer’s Drug Lecanemab
Scientific American

No drug with consistent statistical evidence from clinical trials had ever been found to slow the course of Alzheimer’s disease before the Food and Drug Administration gave its nod this month to lecanemab, which clears the brain of the toxic amyloid protein that has been a primary target for drug developers. Lecanemab, marketed as Leqembi by the companies Eisai and Biogen, is not a cure for Alzheimer’s. Its sometimes serious side effects, modest benefits and yearly price of $26,500 (not yet covered by Medicare) are more than enough to give pause to patients and doctors alike. But the FDA approval marks a milestone for a field that has witnessed numerous failures over the years. Patients with early-stage Alzheimer’s or mild cognitive impairment will quickly find that lecanemab is not a neurological medication that sedates, stimulates or dampens pain. It will not make a person receiving an every-other-week infusion feel smarter. Their memory will not suddenly improve. The drug will just slightly slow the unremitting progression of Alzheimer’s.
See full story at Scientific American