Siemens Healthineers Gets FDA Clearance for Alzheimer’s Disease Analysis Features
itn
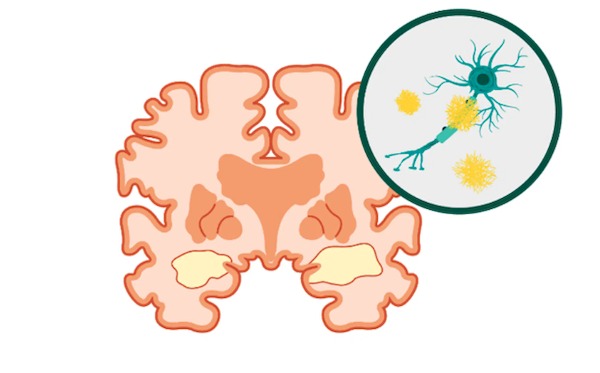
Siemens Healthineers recently received Food and Drug Administration clearance for two new features in its syngo.PET Cortical Analysis software application that use positron emission tomography (PET) to measure protein deposits in the brain associated with Alzheimer’s disease. The first new feature, Centiloid scoring, standardizes the quantification of brain-amyloid plaque in PET scans across three commercially available beta-amyloid PET radiopharmaceuticals used to diagnose Alzheimer’s disease. The other new feature, tau PET quantification, assesses and quantifies the distribution and density of tau protein tangles in the brain from a PET image acquired using the radiopharmaceutical flortaucipir
See full story at itn
Sign up for our newsletter!
Get the latest information and inspirational stories for caregivers, delivered directly to your inbox.